
COVID-19 greatly affected Europe between March and May 2020. Initial reports suggest cancer and haematological malignancies as risk factors for severity and mortality, but the role of allogeneic stem cell transplantation (alloHSCT) remains unclear. The Société Francophone de Greffe de Moelle et Thérapie Cellulaire conducted a multicentre retrospective study of alloHSCT recipients diagnosed with COVID-19. We described the COVID-19 disease characteristics in this population and examined risk factors for severity and mortality.
Data were collected retrospectively from the patients' charts and the ProMISe database. Diagnosis was retained only if a reverse transcription polymerase chain reaction assay test from a nose swab was positive for SARS-CoV-2. Patients were classified as severe if they were transferred to an intensive care unit (ICU) due to COVID-19 or died of COVID-19, and non-severe in other cases. Comparisons of characteristics were performed using student's t-tests and Mann-Whitney U tests for normally and abnormally distributed data, respectively, for continuous variables and χ2 or Fisher's exact tests, when appropriate for categorical variables. Risk factors associated with a severe form of COVID-19 were assessed using both univariate and multivariate logistic regressions. All analyses were performed using SAS version 9.4.6 (SAS Institute Inc., Cary, NC, USA. A two-tailed significance level p<0.05 was used.
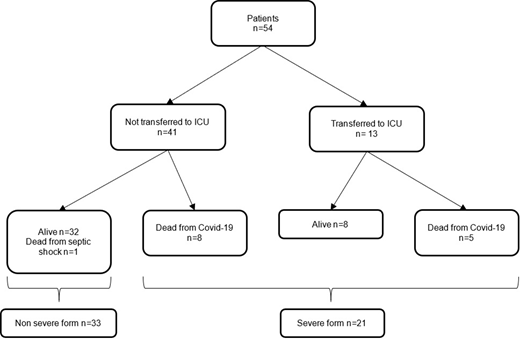
Fifty-four patients were diagnosed, including 21 with severe forms (intensive care transfer and/or death). Haematological characteristics did not vary between patients with severe or non-severe forms of COVID-19. Patients with a severe form of COVID-19 were more likely to be diagnosed earlier after alloHSCT (0.78 vs. 2.1 years, p=0.01), to have comorbidities (80.9% vs. 54.5%, p=0.05) and to receive immunosuppressive treatment (81% vs. 51.5%, p=0.03). Severe COVID-19 patients were more likely to have symptoms at COVID-19 diagnosis (100% vs. 81.8%, p=0.04), especially pneumonia and symptoms other than respiratory or digestive (asthenia, neurological symptoms, myalgia, dysgeusia, skin lesions and arthralgia), and to experience co-infection during the course of the disease (52.4% vs. 21.2%, p= 0.001).
At COVID-19 diagnosis, patients with a non-severe form were more likely to have a higher platelet count (226 G/L vs. 98 G/L, p= 0.01), while other biological characteristics did not vary between the two cohorts. In univariate analysis, shorter time from transplant to COVID-19 (before 211 days, p=0.01), pneumonia (OR 12.21 [95% CI 2.43 - 61.46], p=0.002), symptoms other than pulmonary or digestive (OR 1.21 [95% CI 1.02 - 11.16], p=0.04), immunosuppressive treatment (OR 5.97 [95% CI 0.75 - 47.42], p=0.03) , co-infection (OR 5.84 [95% CI 1.65-20.63], p=0.006) and comorbidity (OR 3.54 [95% CI 0.98-12.83], p=0.05) were associated with severe COVID-19. The only biological parameter associated with severity was a lower platelet count <71G/L (OR 28.00 [95% CI 2.07-379.25]), p=0.008. In multivariate analysis, pneumonia and other symptoms retained a significant association with severe COVID-19.
Thirteen patients died of COVID-19: in univariate analysis, risk factors for death from COVID-19 were similar to the risk factors for severe COVID-19 (i.e. shorter time from alloHSCT, p=0.03; pneumonia, p=0.01; co-infection during the course of COVID-19, p<0.01, and lower platelet count, p<0.01). In multivariate analysis, none of the above mentioned factors remained significantly associated with death from COVID-19.
As SARS-CoV-2 continues to spread internationally, given the lack of vaccine or treatment, alloHSCT recipients should maintain a high level of awareness to avoid contamination.
Dalle:Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria; Bellicum: Consultancy, Honoraria; AbbVie Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Orchard: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; bluebird bio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi-Genzyme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Medac: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Rubio:MSD: Honoraria; Novartis: Honoraria; Neovii: Research Funding; Medac: Consultancy; Gilead: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal